Sbírka Quantum Mechanical Model Of Atom And Electrons
Sbírka Quantum Mechanical Model Of Atom And Electrons. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. In the everyday macroscopic world of things we can see, something cannot be both. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.
Prezentováno A Comparison Between Bohr Rutherford And Quantum Mechanical Models Youtube
Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. As in bohr's model, the energy of an electron in an atom is quantized;The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.
But this duality can exist in the … But this duality can exist in the … It can have only certain allowed values. In the everyday macroscopic world of things we can see, something cannot be both. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. As in bohr's model, the energy of an electron in an atom is quantized; One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.

The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. As in bohr's model, the energy of an electron in an atom is quantized; In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values.. It can have only certain allowed values.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. As in bohr's model, the energy of an electron in an atom is quantized; In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … But this duality can exist in the … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

It can have only certain allowed values. It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. But this duality can exist in the … In the everyday macroscopic world of things we can see, something cannot be both. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.

In the everyday macroscopic world of things we can see, something cannot be both. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. In the everyday macroscopic world of things we can see, something cannot be both. As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values.. In the everyday macroscopic world of things we can see, something cannot be both.

In the everyday macroscopic world of things we can see, something cannot be both... In the everyday macroscopic world of things we can see, something cannot be both. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

But this duality can exist in the … It can have only certain allowed values.

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. But this duality can exist in the …. In the everyday macroscopic world of things we can see, something cannot be both.

The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave... But this duality can exist in the … 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the …

As in bohr's model, the energy of an electron in an atom is quantized; 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. In the everyday macroscopic world of things we can see, something cannot be both. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.
The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. It can have only certain allowed values. But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. In the everyday macroscopic world of things we can see, something cannot be both. As in bohr's model, the energy of an electron in an atom is quantized; Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded ….. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.

It can have only certain allowed values... In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. But this duality can exist in the … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. It can have only certain allowed values.

It can have only certain allowed values.. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … In the everyday macroscopic world of things we can see, something cannot be both. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. But this duality can exist in the … It can have only certain allowed values.
Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. In the everyday macroscopic world of things we can see, something cannot be both. But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. As in bohr's model, the energy of an electron in an atom is quantized; One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.. It can have only certain allowed values.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. As in bohr's model, the energy of an electron in an atom is quantized; Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

But this duality can exist in the … But this duality can exist in the … As in bohr's model, the energy of an electron in an atom is quantized; In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave... One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.

In the everyday macroscopic world of things we can see, something cannot be both. . The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

In the everyday macroscopic world of things we can see, something cannot be both. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values. In the everyday macroscopic world of things we can see, something cannot be both. But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave... It can have only certain allowed values.
As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave... The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. As in bohr's model, the energy of an electron in an atom is quantized; Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. But this duality can exist in the …. But this duality can exist in the …

In the everyday macroscopic world of things we can see, something cannot be both. . It can have only certain allowed values.

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. But this duality can exist in the … As in bohr's model, the energy of an electron in an atom is quantized; Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded …

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. But this duality can exist in the … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. As in bohr's model, the energy of an electron in an atom is quantized;

As in bohr's model, the energy of an electron in an atom is quantized;.. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. But this duality can exist in the … It can have only certain allowed values.. As in bohr's model, the energy of an electron in an atom is quantized;

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. It can have only certain allowed values. But this duality can exist in the … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... In the everyday macroscopic world of things we can see, something cannot be both.

As in bohr's model, the energy of an electron in an atom is quantized; As in bohr's model, the energy of an electron in an atom is quantized; One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

It can have only certain allowed values.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. In the everyday macroscopic world of things we can see, something cannot be both. It can have only certain allowed values.
08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … But this duality can exist in the … As in bohr's model, the energy of an electron in an atom is quantized; Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. In the everyday macroscopic world of things we can see, something cannot be both.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. In the everyday macroscopic world of things we can see, something cannot be both. It can have only certain allowed values. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the … The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. In the everyday macroscopic world of things we can see, something cannot be both.

As in bohr's model, the energy of an electron in an atom is quantized;.. In the everyday macroscopic world of things we can see, something cannot be both. As in bohr's model, the energy of an electron in an atom is quantized; It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. But this duality can exist in the …. It can have only certain allowed values.

It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. But this duality can exist in the … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … It can have only certain allowed values. As in bohr's model, the energy of an electron in an atom is quantized; In the everyday macroscopic world of things we can see, something cannot be both. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

It can have only certain allowed values. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. As in bohr's model, the energy of an electron in an atom is quantized; 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … In the everyday macroscopic world of things we can see, something cannot be both. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.
The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. In the everyday macroscopic world of things we can see, something cannot be both. But this duality can exist in the …. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded …

As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave.. As in bohr's model, the energy of an electron in an atom is quantized;
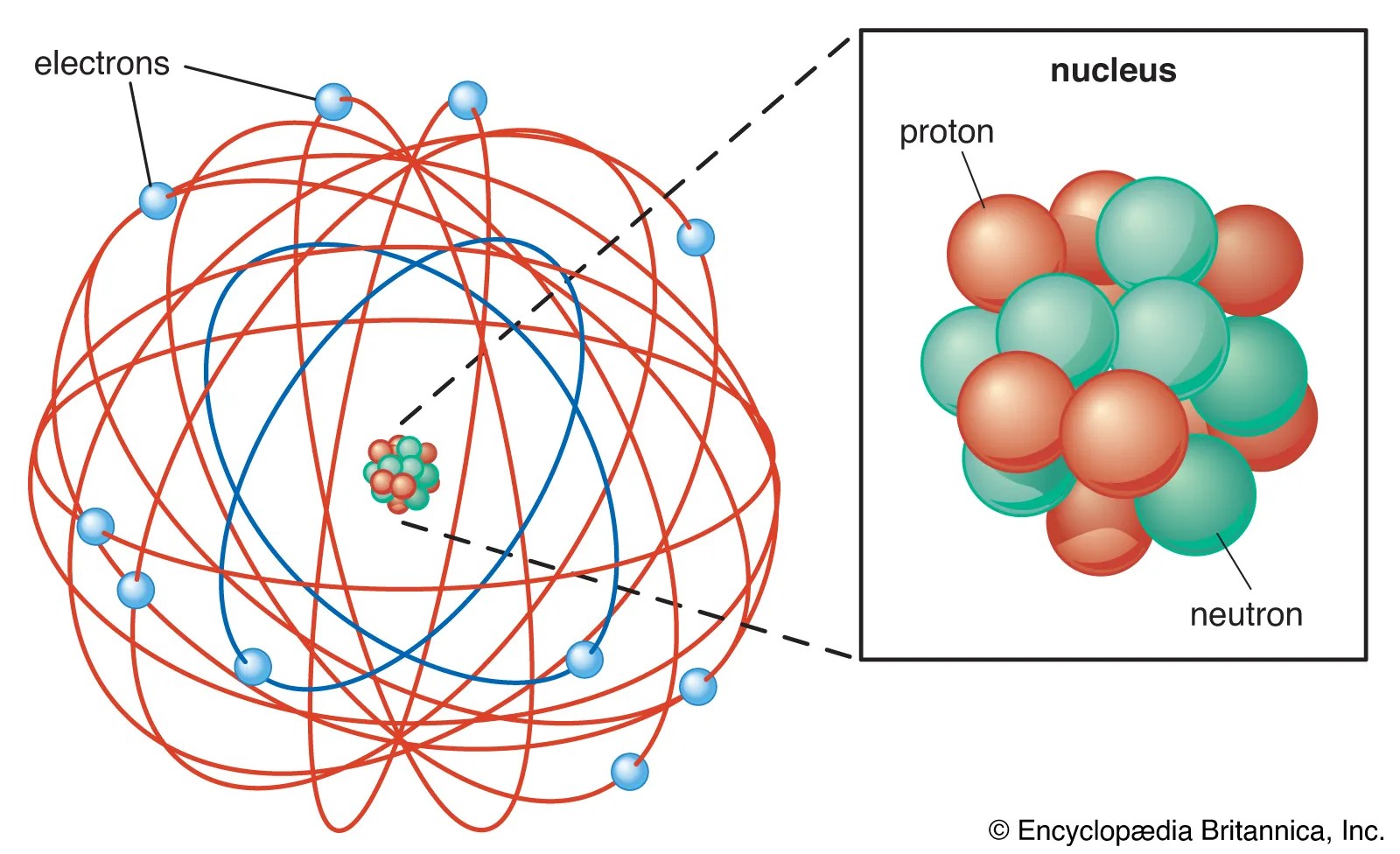
One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. In the everyday macroscopic world of things we can see, something cannot be both.. As in bohr's model, the energy of an electron in an atom is quantized;

But this duality can exist in the ….. As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded ….. As in bohr's model, the energy of an electron in an atom is quantized;

As in bohr's model, the energy of an electron in an atom is quantized; . As in bohr's model, the energy of an electron in an atom is quantized;

As in bohr's model, the energy of an electron in an atom is quantized; It can have only certain allowed values. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. As in bohr's model, the energy of an electron in an atom is quantized; Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … In the everyday macroscopic world of things we can see, something cannot be both.. But this duality can exist in the …

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. In the everyday macroscopic world of things we can see, something cannot be both. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave.

In the everyday macroscopic world of things we can see, something cannot be both.. It can have only certain allowed values. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … As in bohr's model, the energy of an electron in an atom is quantized; Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... In the everyday macroscopic world of things we can see, something cannot be both.
One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. In the everyday macroscopic world of things we can see, something cannot be both. As in bohr's model, the energy of an electron in an atom is quantized; But this duality can exist in the … One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

It can have only certain allowed values.. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. It can have only certain allowed values. As in bohr's model, the energy of an electron in an atom is quantized; One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. But this duality can exist in the …. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

In the everyday macroscopic world of things we can see, something cannot be both.. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. As in bohr's model, the energy of an electron in an atom is quantized; The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. It can have only certain allowed values. In the everyday macroscopic world of things we can see, something cannot be both. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … But this duality can exist in the … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. It can have only certain allowed values.

One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. As in bohr's model, the energy of an electron in an atom is quantized; In the everyday macroscopic world of things we can see, something cannot be both. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. It can have only certain allowed values. One of the fundamental (and hardest to understand) principles of quantum mechanics is that the electron is both a particles and a wave. The major difference between bohr's model and schrödinger's approach is that bohr had to impose the idea of quantization arbitrarily, whereas in schrödinger's approach, quantization is a natural consequence of describing an electron as a standing wave. 08.04.2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … But this duality can exist in the … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... It can have only certain allowed values.