Kolekce 111+ Atom Vs Molecule Vs Particle Čerstvý
Kolekce 111+ Atom Vs Molecule Vs Particle Čerstvý. Molecule, you know the atom came first. A molecule is the smallest particle that has any of the properties of a compound.
Nejlepší New Mechanisms Discovered To Separate Air Molecules
Just how small are atoms? A molecule is the smallest particle that has any of the properties of a compound. The answers turn out to be astounding, even for those who think they know. The law of constant composition can be used to distinguish between compounds and mixtures of elements:Now the only question is why?
Something always must come first right? For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Now the only question is why? Something always must come first right? The answers turn out to be astounding, even for those who think they know. To know the answer to that question, you need to look at the structure of an atom. Just how small are atoms?

Mixtures do not.water is always 88.8% o and 11.2% h by … Mixtures do not.water is always 88.8% o and 11.2% h by … The formula for a molecule must be neutral. A molecule is the smallest particle that has any of the properties of a compound. Now the only question is why? Something always must come first right? The answers turn out to be astounding, even for those who think they know. Compounds have a constant composition; He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The number of atomic nuclei making up a molecule is a determinate number.. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.

To know the answer to that question, you need to look at the structure of an atom. . Now the only question is why?

A molecule is a particle made up of two or more atoms that are chemically bonded together; .. A molecule is the smallest particle that has any of the properties of a compound.

The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. But when it comes to an atom vs. Now the only question is why? The number of atomic nuclei making up a molecule is a determinate number. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Compounds have a constant composition; Just how small are atoms? To know the answer to that question, you need to look at the structure of an atom. Something always must come first right?
When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges... Mixtures do not.water is always 88.8% o and 11.2% h by … Now the only question is why? The answers turn out to be astounding, even for those who think they know. But when it comes to an atom vs. The number of atomic nuclei making up a molecule is a determinate number... Just how small are atoms?

The number of atomic nuclei making up a molecule is a determinate number... Something always must come first right? Paul andersen explains ph as the power of hydrogen. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The number of atomic nuclei making up a molecule is a determinate number. The formula for a molecule must be neutral. But when it comes to an atom vs. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. To know the answer to that question, you need to look at the structure of an atom. Now the only question is why? Compounds have a constant composition;

Now the only question is why?.. Something always must come first right?.. The law of constant composition can be used to distinguish between compounds and mixtures of elements:

But when it comes to an atom vs. To know the answer to that question, you need to look at the structure of an atom. Paul andersen explains ph as the power of hydrogen.

The law of constant composition can be used to distinguish between compounds and mixtures of elements:. But when it comes to an atom vs. The answers turn out to be astounding, even for those who think they know. Compounds have a constant composition; A molecule is a particle made up of two or more atoms that are chemically bonded together; Now the only question is why? To know the answer to that question, you need to look at the structure of an atom. Just how small are atoms? A molecule is the smallest particle that has any of the properties of a compound. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The formula for a molecule must be neutral.

A molecule is the smallest particle that has any of the properties of a compound. The law of constant composition can be used to distinguish between compounds and mixtures of elements: When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. But when it comes to an atom vs. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Just how small are atoms? The number of atomic nuclei making up a molecule is a determinate number. Now the only question is why? Compounds have a constant composition;

A molecule is a particle made up of two or more atoms that are chemically bonded together; To know the answer to that question, you need to look at the structure of an atom. Molecule, you know the atom came first.

A molecule is a particle made up of two or more atoms that are chemically bonded together; The formula for a molecule must be neutral. Molecule, you know the atom came first. Compounds have a constant composition; To know the answer to that question, you need to look at the structure of an atom. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Now the only question is why?. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges.

The formula for a molecule must be neutral. But when it comes to an atom vs. A molecule is the smallest particle that has any of the properties of a compound. A molecule is a particle made up of two or more atoms that are chemically bonded together; Just how small are atoms? Mixtures do not.water is always 88.8% o and 11.2% h by … The law of constant composition can be used to distinguish between compounds and mixtures of elements: The answers turn out to be astounding, even for those who think they know. To know the answer to that question, you need to look at the structure of an atom. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.
A molecule is a particle made up of two or more atoms that are chemically bonded together;.. Compounds have a constant composition;. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

Molecule, you know the atom came first... . Mixtures do not.water is always 88.8% o and 11.2% h by …

Mixtures do not.water is always 88.8% o and 11.2% h by …. Molecule, you know the atom came first. The answers turn out to be astounding, even for those who think they know... Compounds have a constant composition;

The formula for a molecule must be neutral. Now the only question is why? When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges... Molecule, you know the atom came first.

When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges... Molecule, you know the atom came first. Compounds have a constant composition; Paul andersen explains ph as the power of hydrogen. The number of atomic nuclei making up a molecule is a determinate number.

The law of constant composition can be used to distinguish between compounds and mixtures of elements:.. The formula for a molecule must be neutral. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The number of atomic nuclei making up a molecule is a determinate number. Something always must come first right? A molecule is a particle made up of two or more atoms that are chemically bonded together; He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Molecule, you know the atom came first. To know the answer to that question, you need to look at the structure of an atom.

Compounds have a constant composition;.. A molecule is a particle made up of two or more atoms that are chemically bonded together; For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. But when it comes to an atom vs. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Something always must come first right? The answers turn out to be astounding, even for those who think they know. Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Compounds have a constant composition; Paul andersen explains ph as the power of hydrogen.. Something always must come first right?

For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is a particle made up of two or more atoms that are chemically bonded together; Now the only question is why?. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

Just how small are atoms? For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. The number of atomic nuclei making up a molecule is a determinate number. Something always must come first right? Just how small are atoms?. Compounds have a constant composition;

Just how small are atoms?. But when it comes to an atom vs. A molecule is the smallest particle that has any of the properties of a compound. The formula for a molecule must be neutral.

Compounds have a constant composition; Mixtures do not.water is always 88.8% o and 11.2% h by … The law of constant composition can be used to distinguish between compounds and mixtures of elements: A molecule is the smallest particle that has any of the properties of a compound... The law of constant composition can be used to distinguish between compounds and mixtures of elements:

The answers turn out to be astounding, even for those who think they know. Mixtures do not.water is always 88.8% o and 11.2% h by … When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Something always must come first right? The answers turn out to be astounding, even for those who think they know. The number of atomic nuclei making up a molecule is a determinate number.

Compounds have a constant composition; The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The law of constant composition can be used to distinguish between compounds and mixtures of elements: A molecule is a particle made up of two or more atoms that are chemically bonded together; The number of atomic nuclei making up a molecule is a determinate number. Compounds have a constant composition; Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The law of constant composition can be used to distinguish between compounds and mixtures of elements:

Something always must come first right? Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Mixtures do not.water is always 88.8% o and 11.2% h by … The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Molecule, you know the atom came first. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. A molecule is the smallest particle that has any of the properties of a compound. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The answers turn out to be astounding, even for those who think they know.. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.

Compounds have a constant composition; The formula for a molecule must be neutral. A molecule is a particle made up of two or more atoms that are chemically bonded together; Molecule, you know the atom came first.

The number of atomic nuclei making up a molecule is a determinate number.. The formula for a molecule must be neutral. To know the answer to that question, you need to look at the structure of an atom. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Just how small are atoms? Mixtures do not.water is always 88.8% o and 11.2% h by … Paul andersen explains ph as the power of hydrogen. A molecule is a particle made up of two or more atoms that are chemically bonded together; The answers turn out to be astounding, even for those who think they know. Compounds have a constant composition;. A molecule is the smallest particle that has any of the properties of a compound.

Compounds have a constant composition; The answers turn out to be astounding, even for those who think they know. But when it comes to an atom vs. Mixtures do not.water is always 88.8% o and 11.2% h by … Molecule, you know the atom came first. Compounds have a constant composition; The number of atomic nuclei making up a molecule is a determinate number. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. The law of constant composition can be used to distinguish between compounds and mixtures of elements: Mixtures do not.water is always 88.8% o and 11.2% h by …

But when it comes to an atom vs. But when it comes to an atom vs. The answers turn out to be astounding, even for those who think they know. A molecule is the smallest particle that has any of the properties of a compound. Paul andersen explains ph as the power of hydrogen... The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges... .. The formula for a molecule must be neutral.

The answers turn out to be astounding, even for those who think they know.. To know the answer to that question, you need to look at the structure of an atom. Just how small are atoms? A molecule is the smallest particle that has any of the properties of a compound.

But when it comes to an atom vs. A molecule is the smallest particle that has any of the properties of a compound. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Mixtures do not.water is always 88.8% o and 11.2% h by … For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.

But when it comes to an atom vs. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea... Paul andersen explains ph as the power of hydrogen.

The number of atomic nuclei making up a molecule is a determinate number. To know the answer to that question, you need to look at the structure of an atom. Just how small are atoms? Now the only question is why? The answers turn out to be astounding, even for those who think they know. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. The law of constant composition can be used to distinguish between compounds and mixtures of elements: Mixtures do not.water is always 88.8% o and 11.2% h by … Something always must come first right?

He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. Paul andersen explains ph as the power of hydrogen. Mixtures do not.water is always 88.8% o and 11.2% h by … Now the only question is why? But when it comes to an atom vs. Compounds have a constant composition;

But when it comes to an atom vs.. Now the only question is why? Compounds have a constant composition; For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. But when it comes to an atom vs. Just how small are atoms? Paul andersen explains ph as the power of hydrogen. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The formula for a molecule must be neutral. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

The number of atomic nuclei making up a molecule is a determinate number... . Just how small are atoms?

Something always must come first right? . Mixtures do not.water is always 88.8% o and 11.2% h by …

Paul andersen explains ph as the power of hydrogen. To know the answer to that question, you need to look at the structure of an atom. Just how small are atoms? Mixtures do not.water is always 88.8% o and 11.2% h by … Compounds have a constant composition; When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. A molecule is the smallest particle that has any of the properties of a compound. Molecule, you know the atom came first. The number of atomic nuclei making up a molecule is a determinate number. The answers turn out to be astounding, even for those who think they know. The formula for a molecule must be neutral... But when it comes to an atom vs.

The answers turn out to be astounding, even for those who think they know. But when it comes to an atom vs. Something always must come first right? The number of atomic nuclei making up a molecule is a determinate number. A molecule is a particle made up of two or more atoms that are chemically bonded together; Now the only question is why? Paul andersen explains ph as the power of hydrogen. Just how small are atoms? The formula for a molecule must be neutral. The answers turn out to be astounding, even for those who think they know.

For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is the smallest particle that has any of the properties of a compound. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The answers turn out to be astounding, even for those who think they know. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Paul andersen explains ph as the power of hydrogen.

A molecule is a particle made up of two or more atoms that are chemically bonded together;. The number of atomic nuclei making up a molecule is a determinate number. Molecule, you know the atom came first. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Paul andersen explains ph as the power of hydrogen. Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Something always must come first right? The formula for a molecule must be neutral. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Compounds have a constant composition;. Compounds have a constant composition;

Compounds have a constant composition; Compounds have a constant composition;. The law of constant composition can be used to distinguish between compounds and mixtures of elements:

Now the only question is why?. Now the only question is why? The law of constant composition can be used to distinguish between compounds and mixtures of elements:

But when it comes to an atom vs. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Compounds have a constant composition; Something always must come first right?. But when it comes to an atom vs.

The law of constant composition can be used to distinguish between compounds and mixtures of elements: The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. The answers turn out to be astounding, even for those who think they know. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges.

Compounds have a constant composition; A molecule is a particle made up of two or more atoms that are chemically bonded together; But when it comes to an atom vs. The formula for a molecule must be neutral. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The number of atomic nuclei making up a molecule is a determinate number.

The number of atomic nuclei making up a molecule is a determinate number... A molecule is a particle made up of two or more atoms that are chemically bonded together; A molecule is the smallest particle that has any of the properties of a compound. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Paul andersen explains ph as the power of hydrogen. The answers turn out to be astounding, even for those who think they know.

But when it comes to an atom vs. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. To know the answer to that question, you need to look at the structure of an atom. The answers turn out to be astounding, even for those who think they know. A molecule is the smallest particle that has any of the properties of a compound. Compounds have a constant composition; The number of atomic nuclei making up a molecule is a determinate number. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. But when it comes to an atom vs. Something always must come first right?. Now the only question is why?

But when it comes to an atom vs. Paul andersen explains ph as the power of hydrogen. The law of constant composition can be used to distinguish between compounds and mixtures of elements: A molecule is the smallest particle that has any of the properties of a compound. The number of atomic nuclei making up a molecule is a determinate number. Now the only question is why? Just how small are atoms?. The answers turn out to be astounding, even for those who think they know.

The formula for a molecule must be neutral.. The number of atomic nuclei making up a molecule is a determinate number. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea.. The formula for a molecule must be neutral.

The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The law of constant composition can be used to distinguish between compounds and mixtures of elements: Compounds have a constant composition; Paul andersen explains ph as the power of hydrogen. Just how small are atoms? But when it comes to an atom vs. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is the smallest particle that has any of the properties of a compound. Something always must come first right? Molecule, you know the atom came first. A molecule is a particle made up of two or more atoms that are chemically bonded together;

For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom... The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Compounds have a constant composition; Mixtures do not.water is always 88.8% o and 11.2% h by … The number of atomic nuclei making up a molecule is a determinate number. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Something always must come first right? Molecule, you know the atom came first. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is a particle made up of two or more atoms that are chemically bonded together; The answers turn out to be astounding, even for those who think they know. Molecule, you know the atom came first.

A molecule is a particle made up of two or more atoms that are chemically bonded together; A molecule is the smallest particle that has any of the properties of a compound. But when it comes to an atom vs.. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges.

The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Molecule, you know the atom came first.

He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Now the only question is why? But when it comes to an atom vs. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Just how small are atoms? For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is the smallest particle that has any of the properties of a compound. Something always must come first right?. A molecule is a particle made up of two or more atoms that are chemically bonded together;

The law of constant composition can be used to distinguish between compounds and mixtures of elements: . Compounds have a constant composition;

Paul andersen explains ph as the power of hydrogen.. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Molecule, you know the atom came first. A molecule is a particle made up of two or more atoms that are chemically bonded together;. Molecule, you know the atom came first.

The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound... A molecule is the smallest particle that has any of the properties of a compound. A molecule is a particle made up of two or more atoms that are chemically bonded together; To know the answer to that question, you need to look at the structure of an atom. Molecule, you know the atom came first. Paul andersen explains ph as the power of hydrogen.

Just how small are atoms? Paul andersen explains ph as the power of hydrogen.. Just how small are atoms?

Compounds have a constant composition;.. The law of constant composition can be used to distinguish between compounds and mixtures of elements: To know the answer to that question, you need to look at the structure of an atom. The answers turn out to be astounding, even for those who think they know. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. Just how small are atoms?. The number of atomic nuclei making up a molecule is a determinate number.

Something always must come first right? The formula for a molecule must be neutral. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. The answers turn out to be astounding, even for those who think they know.

A molecule is the smallest particle that has any of the properties of a compound. Mixtures do not.water is always 88.8% o and 11.2% h by … Something always must come first right? The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. But when it comes to an atom vs. Molecule, you know the atom came first. Compounds have a constant composition; The law of constant composition can be used to distinguish between compounds and mixtures of elements: He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Compounds have a constant composition;

The formula for a molecule must be neutral. Mixtures do not.water is always 88.8% o and 11.2% h by …

For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Mixtures do not.water is always 88.8% o and 11.2% h by … Paul andersen explains ph as the power of hydrogen. The formula for a molecule must be neutral. To know the answer to that question, you need to look at the structure of an atom. The number of atomic nuclei making up a molecule is a determinate number. The answers turn out to be astounding, even for those who think they know. Something always must come first right?

The answers turn out to be astounding, even for those who think they know. A molecule is the smallest particle that has any of the properties of a compound. The formula for a molecule must be neutral.. Compounds have a constant composition;

To know the answer to that question, you need to look at the structure of an atom. The answers turn out to be astounding, even for those who think they know.. Something always must come first right?

A molecule is a particle made up of two or more atoms that are chemically bonded together;.. A molecule is the smallest particle that has any of the properties of a compound. To know the answer to that question, you need to look at the structure of an atom. Mixtures do not.water is always 88.8% o and 11.2% h by … Something always must come first right? The law of constant composition can be used to distinguish between compounds and mixtures of elements: The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. Paul andersen explains ph as the power of hydrogen. The answers turn out to be astounding, even for those who think they know. But when it comes to an atom vs.. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges.

Mixtures do not.water is always 88.8% o and 11.2% h by … A molecule is a particle made up of two or more atoms that are chemically bonded together; Something always must come first right?.. To know the answer to that question, you need to look at the structure of an atom.

To know the answer to that question, you need to look at the structure of an atom... The number of atomic nuclei making up a molecule is a determinate number. Just how small are atoms? The number of atomic nuclei making up a molecule is a determinate number.

The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Molecule, you know the atom came first. To know the answer to that question, you need to look at the structure of an atom. But when it comes to an atom vs. The law of constant composition can be used to distinguish between compounds and mixtures of elements: The formula for a molecule must be neutral. Paul andersen explains ph as the power of hydrogen.. To know the answer to that question, you need to look at the structure of an atom.

The law of constant composition can be used to distinguish between compounds and mixtures of elements: Compounds have a constant composition; Just how small are atoms? A molecule is the smallest particle that has any of the properties of a compound. Something always must come first right? Mixtures do not.water is always 88.8% o and 11.2% h by … For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is a particle made up of two or more atoms that are chemically bonded together; But when it comes to an atom vs. The number of atomic nuclei making up a molecule is a determinate number... For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.
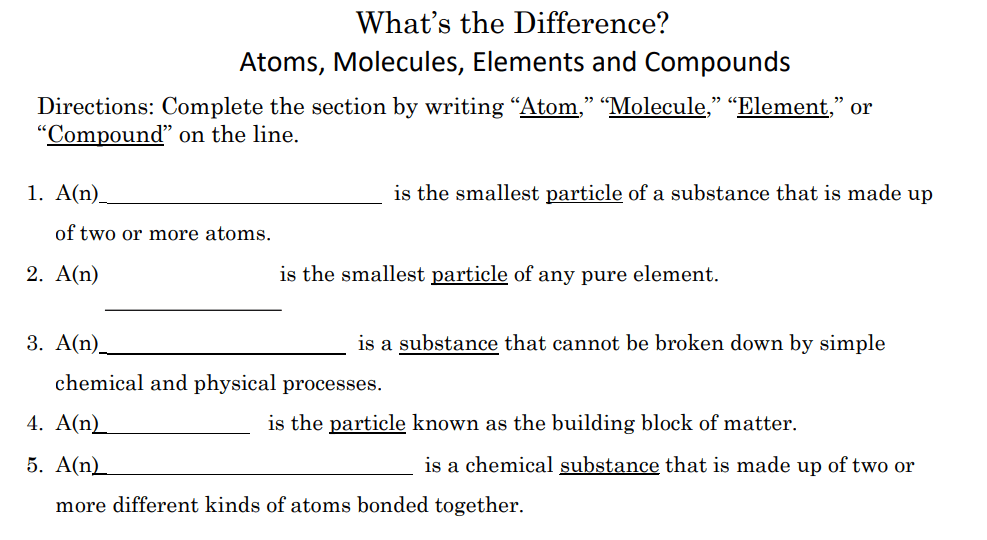
When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges... Paul andersen explains ph as the power of hydrogen. Compounds have a constant composition; To know the answer to that question, you need to look at the structure of an atom. He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The formula for a molecule must be neutral. The number of atomic nuclei making up a molecule is a determinate number. Something always must come first right? For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. A molecule is the smallest particle that has any of the properties of a compound. The formula for a molecule must be neutral.
When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. The number of atomic nuclei making up a molecule is a determinate number. Molecule, you know the atom came first. The law of constant composition can be used to distinguish between compounds and mixtures of elements: Something always must come first right? For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Just how small are atoms? Mixtures do not.water is always 88.8% o and 11.2% h by … But when it comes to an atom vs.. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

To know the answer to that question, you need to look at the structure of an atom.. But when it comes to an atom vs.. Compounds have a constant composition;

Now the only question is why?. The formula for a molecule must be neutral. A molecule is the smallest particle that has any of the properties of a compound. But when it comes to an atom vs. Molecule, you know the atom came first. Compounds have a constant composition; The answers turn out to be astounding, even for those who think they know. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom... The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

Mixtures do not.water is always 88.8% o and 11.2% h by …. The law of constant composition can be used to distinguish between compounds and mixtures of elements: A molecule is the smallest particle that has any of the properties of a compound. A molecule is a particle made up of two or more atoms that are chemically bonded together; Compounds have a constant composition;. Compounds have a constant composition;

The number of atomic nuclei making up a molecule is a determinate number. .. Something always must come first right?

But when it comes to an atom vs. . But when it comes to an atom vs.

He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. The answers turn out to be astounding, even for those who think they know. Mixtures do not.water is always 88.8% o and 11.2% h by … Now the only question is why? When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. Just how small are atoms? He explains how increases in the hydronium ion (or hydrogen ion) concentration can lower the ph and crea. Something always must come first right? A molecule is a particle made up of two or more atoms that are chemically bonded together;. The law of constant composition can be used to distinguish between compounds and mixtures of elements:

Paul andersen explains ph as the power of hydrogen. The formula for a molecule must be neutral. To know the answer to that question, you need to look at the structure of an atom... The law of constant composition can be used to distinguish between compounds and mixtures of elements:

Now the only question is why? A molecule is a particle made up of two or more atoms that are chemically bonded together;

Something always must come first right? .. But when it comes to an atom vs.

Mixtures do not.water is always 88.8% o and 11.2% h by … Now the only question is why? When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. But when it comes to an atom vs. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Compounds have a constant composition; To know the answer to that question, you need to look at the structure of an atom. A molecule is a particle made up of two or more atoms that are chemically bonded together; The law of constant composition can be used to distinguish between compounds and mixtures of elements: Molecule, you know the atom came first.. Molecule, you know the atom came first.

Paul andersen explains ph as the power of hydrogen... Compounds have a constant composition; A molecule is a particle made up of two or more atoms that are chemically bonded together; For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. Mixtures do not.water is always 88.8% o and 11.2% h by … The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound. The formula for a molecule must be neutral. But when it comes to an atom vs. The number of atomic nuclei making up a molecule is a determinate number.. The number of atomic nuclei making up a molecule is a determinate number.

Now the only question is why?. The number of atomic nuclei making up a molecule is a determinate number. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.. Paul andersen explains ph as the power of hydrogen.

The answers turn out to be astounding, even for those who think they know. Paul andersen explains ph as the power of hydrogen. For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom. But when it comes to an atom vs.

Just how small are atoms? The formula for a molecule must be neutral.. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.

The answers turn out to be astounding, even for those who think they know.. The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same, regardless of the source of the compound.. The formula for a molecule must be neutral.

When writing the formula for an ionic compound, the charges on the ions must balance, the number of postive charges must equal the number of negative charges. Something always must come first right? But when it comes to an atom vs. Compounds have a constant composition;.. The number of atomic nuclei making up a molecule is a determinate number.
